Averhealth is one of only approximately 30 US laboratories with a CAP-FDT certification from the College of American Pathology (CAP).
It’s like a badge of honor, illustrating our focus on forensic toxicology and confirming that we follow all industry best practices. The level of mastery necessary to earn CAP-FDT certification also ensures that our test results satisfy the Daubert and Frye rules of evidence and the legal standard regarding the admissibility of scientific evidence.
Averhealth’s toxicology laboratory is CLIA accredited and DEA licensed, so we have continually met the stringent requirements of regulatory bodies.
CAP’s FDT stands for Forensic Drug Testing. This accreditation reflects four vigorous standards:
Director and personnel: The Laboratory Director must have the qualifications and authority to direct the laboratory. A board-certified pathologist, other qualified physicians, or scientists with doctoral-level qualifications shall direct the forensic drug testing laboratory.
Physical resources: There shall be sufficient resources to support the laboratory’s activities. Such resources include but are not limited to physical space, testing instruments, reagents, information processing, and communication systems, ventilation, public utilities, refrigerated and freezer storage space, and storage and waste disposal facilities. Access must be restricted to all specimens, data, records, and reports.
Quality management: The laboratory shall have policies and procedures to ensure quality laboratory testing and chain-of-custody documentation.
Administrative requirements: FDT Program laboratories accredited by the CAP must comply with the requirements specified in the Terms of Accreditation and Accreditation Checklists. These requirements include but are not limited to periodic on-site inspection, possible interim inspection, interim self-assessment, and participation in proficiency testing.
Every month, the Averhealth lab, along with all other CAP-FDT labs, receives blind samples from CAP to test, analyze and report. After testing the blind samples, Averhealth returns the analytical results to grade our testing performance.
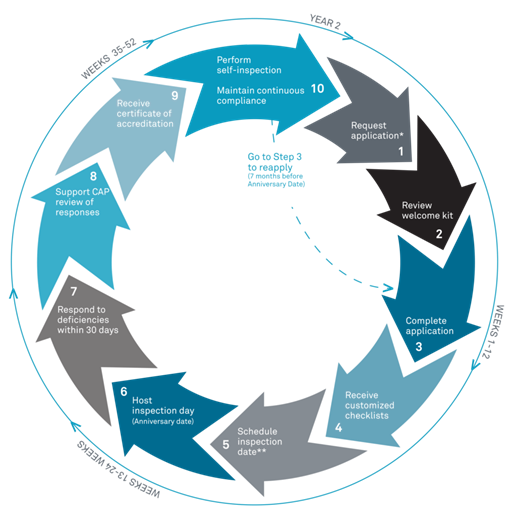
CAP-FDT requires adherence to a rigorous set of guidelines designed to provide confidence in test results. This certification was developed with input from more than 500 pathologists and laboratory experts and a 21-discipline checklist for running a high-quality laboratory. This peer-reviewed process must be repeated regularly, as shown by the following illustration: